Regenerative Therapy
Exosomes, the active vesicle of the stem cell, have been shown to be efficacious in a number of studies related to repair of peripheral nerve damage, traumatic brain injury, accelerated wound healing, tissue repair, bone fusion, skin regeneration, cartilage regeneration, treatment for injured ligaments and tendons, osteoarthritis, bone regeneration, vaginal rejuvenation, chronic inflammation, Long COVID, and many other conditions, especially related to inflammation.
Exosomes pass through the blood brain barrier.
Exosomes are NOT living cells. Therefore they do not need to be “matched” to patients.
Compared to stem cells, exosome therapy is free from DNA and therefore represents a treatment with lower risk. Exosomes are not living cells. Studies have show that therapy with exosomes show similar or better results when compared to stem cell treatment. Based on this information, it is though that stem cell treatment facilitates its beneficial effects through the secretions of exosomes.
Find the Right Solution For You
Exosomes
Exosomes are small vesicles that are secreted by stem cells. They contain large amounts of growth factors, cytokines, and other substances with high regenerative potential that can be transferred to other cells due to their unique structure. This structure allows the exosomes to fuse with other cells in the body and to release their content into other cells. The contents of the exosomes such as cytokines, growth factors, and mRNA can then help the cell heal itself due to the actions of the transferred signaling molecules.
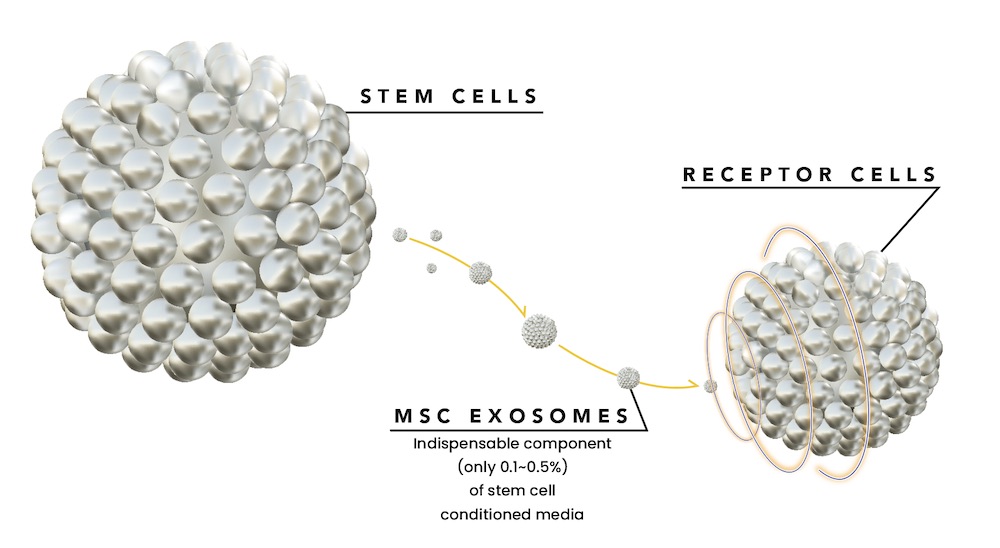
Contrary to stem cells, exosomes do not replicate, do not contain DNA, and do not elicit an immune response, resulting in a safer product compared to stem cells. By definition, exosome products are cell-free and do not contain any live cells. Our team has been in the regeneration business for 11 years. Specifically, to develop this product, not to have it sold under the table but rather as a true pharmaceutical-grade drug. Our product meets purity, potency, consistency, and safety standards set by the FDA’s 351(a) regulations. Due to the high medical risks of using live stem cells, they have been highly regulated. Currently, the only legal way to use live stem cells is if they are derived from the patient themselves or are not manipulated in any way and used as a tissue transplant. These current methods of using live stem cells have major shortcomings beyond the health risk.
First, any tissue that is used as a transplant is limited in its application in only being able to be applied to similar tissue (placenta membranes may only be used on similar skin type tissues).
Second, any self-derived stem cells are subject to degradation due to aging. At the age of 40, a person’s stem cells begin to lose effectiveness. By the age of 60, the age of most patients needing stem cell treatments, the stem cells are barely effective, if at all.
We combat this by using a placenta from a new birth and extracting the mesenchymal stem cells. This allows us to use the newest and most potent stem cells possible. We then screen and culture them in a lab to control the safety and efficacy of the cells. We then immerse them in the appropriate medium which allows them to secrete exosomes with consistent and high quantities of regenerative factors. Finally, we extract the exosomes and analyze them through batch testing at which point they are made available for research purposes.
IMPORTANT NOTICE:
Stem cell-derived exosomes are not currently approved by the FDA for use in medical treatments. They are exclusively sold for research purposes in various areas, including:
- Arthritis
- Bone fusion & regeneration
- Burns/wounds
- Cartilage regeneration
- Cerebral palsy
- COVID-19 long-haulers
- Dementia
- Dermatology / Aesthetics
- Glaucoma
- Hair loss
- Injured
- ligaments/tendons
- Multiple sclerosis
- Orthopedics
- Osteoarthritis
- Peripheral nerve damage
- Respiratory
- Sexual dysfunction
- Skin regeneration
- Spinal cord injuries
- Stroke
- Tissue repair
- Traumatic brain injury
- Vaginal regeneration
- Wound care
Get in touch
We will be in touch shortly
We’d love to hear from you! Whether you have questions, or feedback, or need assistance, our team is here to help. Reach out, and we’ll get back to you promptly.
Mailing Address
PO Box 26048 Scottsdale AZ 85255
Email us
info@matrixbiologics.com
Call us
602-480-0486